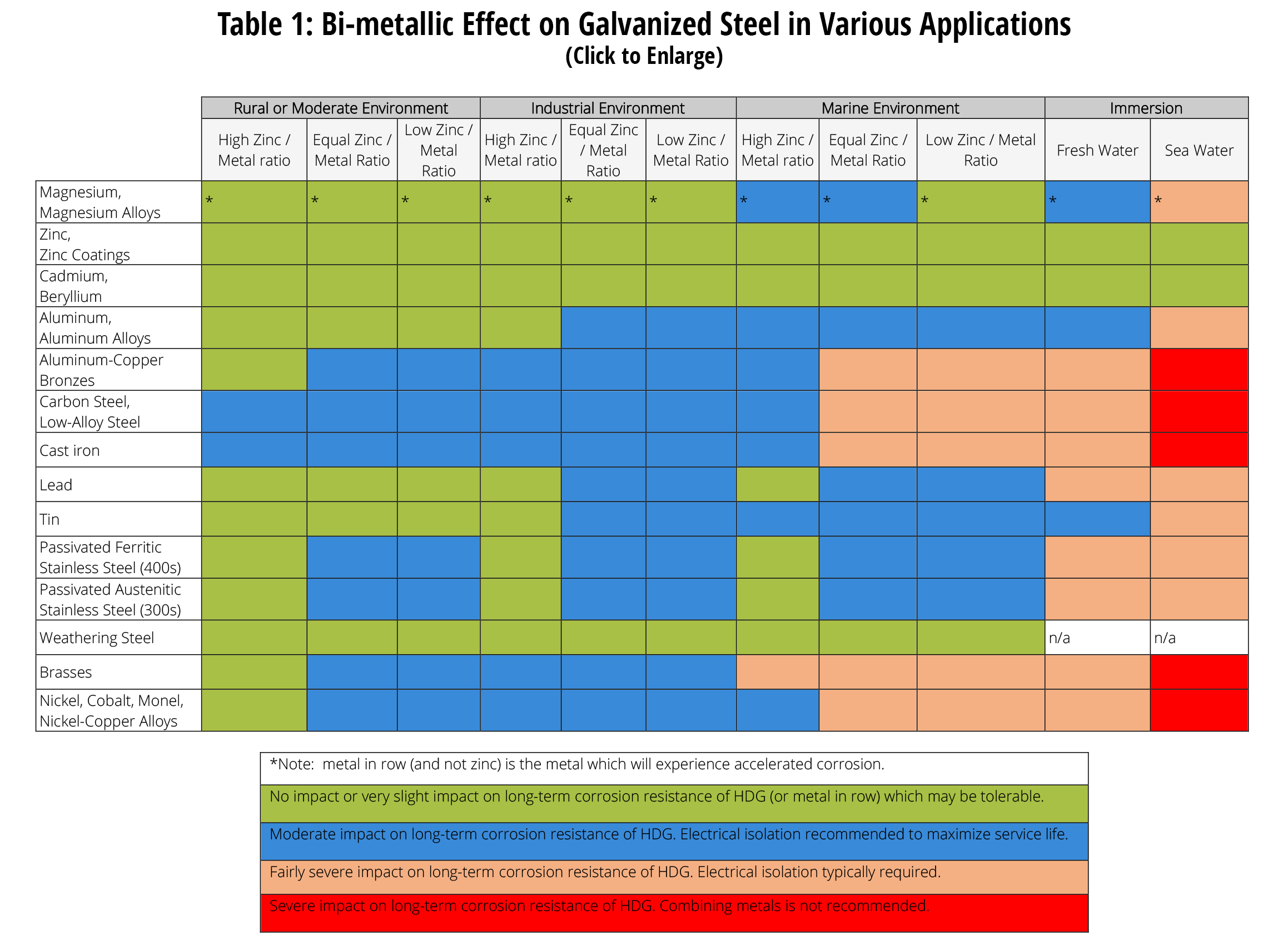
When two different metals are in contact in a corrosive environment one of the metals experiences accelerated galvanic corrosion while the other metal remains.
Galvanized steel sheet electrolysis.
The galvanized steel anodes should not be used in electrolysis.
This is not to say that no form of corrosion will ever occur though.
If you break the conductive metal circuit between the copper and galvanized pipe galvanic corrosion electrolysis does not occur.
If zinc and or other coat metals get into the electrolyte they will form compounds which in their turn will contribute alloyed metals to the cathode some plating of the iron object being de rusted may occur.
Stainless steel and galvanized materials often are found together in the industry with applications such as galvanized fasteners stainless steel pressure vessels and roof and siding panels.
Galvanized steel also can be coated with nickel copper etc.
Hot dip galvanized steel is well suited for use in a variety of environments and fabrications and sometimes is placed in contact with different metals including among others stainless steel aluminum copper and weathering steel.
Galvanized steel sheet plates are intended for use where greater corrosion protection is required without painting.